
Objectives
The objectives of this article are to give the reader a greater appreciation for carbon steel and what it can do, to give a rudimentary explanation for how heat treatment works and to give some examples. The objective is not to give a detailed metallurgical explanation of the exact physical mechanisms involved in heat treatment or step-by-step instructions on how to do it.
The Miracle
A piece of iron, uncombined with anything else, is nothing extraordinary. It bends, rusts and is decidedly inferior to bronze in strength. The miracle happens when you add from 0.3 to 0.95 percent carbon to the iron and then heat treat it. It becomes hard enough to saw through bronze the way bronze could cut through meat. The simple expedient of combining two of nature's commonest elements, iron and carbon to form a material that is relatively easy to shape yet can make a tool of unprecedented durability and sharpness is one of the most underappreciated technologies on which modern civilization is based. This was one of our great labor saving inventions. The ability to cut, saw'or chop our way through wood, meat or even other metals quickly and precisely freed up huge amounts of time for our ancient ancestors to do much more. Yet today we take all this for granted. I have spoken with primitive technology buffs who have made bow and arrows using nothing but stone age tools. It takes for flamin' ever!
A similar technological breakthrough happened much earlier when our stone age ancestors learned to cook their food. Without cooking most of what we eat would be digested poorly and require a huge amount of chewing. As a point of reference gorillas spend about eight hours a day just chewing great masses of low calorie vegetable matter. Less time chewing means more time and energy to develop human speech, technology, art and getting into mischief. Learn more about how cooked food civilized us by reading Fire - The Spark that Ignited Human Evolution by Francis Burton and Catching Fire: How Cooking Made Us Human by Richard Wrangham.
The Metallurgy
Take some iron ore, primarily iron oxide, and some carbon, in the form of charcoal or coke and heat them up together. Get the combination hot enough and the oxygen in the iron ore combines with the carbon and you get metallic iron and carbon dioxide. If you get it barely hot enough you get a spongy mass of almost pure iron that can be shaped by pounding. If you get it really, really hot you get molten iron and an excessive amount of the carbon is dissolved in the iron, resulting in cast iron. The challenge is to obtain the desired amount of carbon in the iron so it can be heat treated to form a tool or weapon with superior edge-holding capability and sharpness. Too little carbon and it won't harden, too much and you get cheap, brittle and easily melted cast iron.
What is the difference between soft and hard? A relatively soft metal such as copper, aluminum, iron or even lead consists of an aggregation of small randomly oriented crystals. Each little crystal is a regular array of atoms stacked together and holding on to each other. The atoms can shift position, letting go of their nearest neighbors and then reattaching to a new set of neighboring atoms. That process allows a piece of metal to bend without breaking.
When you add carbon atoms to iron they tend to be located inside the crystal lattice in places where they fit easily and have relatively little influence on the strength of the iron-carbon alloy known as carbon steel. If only we could stick those carbon atoms in locations which would prevent the iron atoms from shifting from one location to another. That's where the magic occurs. When you heat carbon steel above a certain temperature a very minor change in its crystal structure happens that has profound consequences. By making a very small shift in position the carbon atoms are relocated to places where they normally wouldn't fit. If only they would stay there then they would prevent the iron atoms from shifting and we would have a much harder material. What happens next is one of the miracles on which modern civilization is based. Simply cool the carbon steel fast enough (quenching) and the carbon atoms remain locked in place and you have a material that is orders of magnitude harder. The knives, axes, razors, automobile gears that we all take for granted depend on this miracle of heat treatment.
Dimensional Change
When you crowd the carbon atoms into locations where they normally wouldn't fit the steel expands. One consequence of this is the graceful curve of the Japanese samurai sword. When the back of the sword remains soft and the edge is hardened then the steel in the edge region is expanded, curving the blade upward. An unfortunate situation happens if there are residual stresses in a blade or if one side of the blade is quenched more rapidly than the other. The blade warps. Then you must use differential tempering before trying to remove the warp.
Hardness
Hardness can be measured in a couple of ways. The hardness of minerals is quantified by what will scratch what. Flint will scratch limestone so flint is harder than limestone. Diamond will scratch flint so it is harder. That list of minerals in ascending order of hardness is called the Moh's scale. Another definition of hardness is 'resistance to penetration'. Many instruments have been developed to measure hardness of materials ranging from rubber to silicon carbide. Hardness of wood is defined as the force needed to depress a 0.444 diameter steel sphere half its diameter in a direction perpendicular to the grain. For cutting tools such as steel knives the Rockwell C scale is used to indicate hardness. That is done by pressing a tiny diamond with- a tightly specified shape into the surface with a specific force. The depth of penetration is a measure of the hardness The inexpensive paring knives in your kitchen probably have a hardness of around Rc56 on that scale. A straight razor might have a hardness of Rc64. Note that going from 56 to 64 makes a huge difference in edge holding capability.
Experience with 1095 Steel
The designation 1095 simply means that it is a plain carbon steel with nominally 0.95 % carbon. The Precision Steel Warehouse catalog lists it as a 'spring steel'. In addition to the carbon it also contains 0.45 % manganese. The manganese is added to improve the 'hardenability'. Earlier I said that steel would harden if cooled 'quickly'. How quickly is quick enough? Remember that the interior of a thick piece of steel cools more slowly than the surface. If the interior cools too slowly then it will not harden, great internal stress will be present and the piece is susceptible to breaking. Adding manganese means that it doesn't have to cool quite as quickly to harden, making it possible to do through-hardening on thicker pieces.
Table 2 compares the properties of 1095 steel with 1050 steel containing only 0.5 % of carbon. It is clear that 1095 has superior hardness and tensile strength over the entire tempering range.
Is improved hardenability always desirable? Not necessarily. Japanese swords are differentially hardened with the back of the sword soft and the cutting edge hard This is achieved by putting a thick clay coating on the region that is to remain soft. When the sword is heated red hot and quenched in water the clay coat prevents the back from cooling quickly. Consequently the swordsmith must use a steel whose hardness depends strongly on cooling rate. Just the opposite of what manganese does. Some bladesmiths do, however, successfully do this type of differential tempering with 1095 steel.
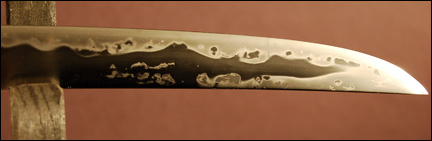
Is it possible to quench the steel too quickly? Unfortunately, yes. 1095 is designated as an 'oil quench' steel meaning that the best way to quench it is in oil. I tried quenching some 1095 blades in water. Bad idea! Because of its heat transfer properties water cools the steel too quickly and results in cracking. Look at Figure 1 and learn from my mistake. The cooling rate was also exacerbated by the thinness of the blades (.05 inch = 1.27 mm). What's also happening when you quench the.steel? When you cram the carbon atoms into locations where they normally wouldn't fit the crystal lattice expands. More carbon atoms squeezed into tight locations means more expansion. If because of differences in cooling rate, part of the blade expands more than another part you get great internal stress that can exceed the strength of the material and the blade cracks. Oh shucks!
Tempering, Differential and Otherwise
Hardness after quenching is very high but unfortunately it is also accompanied by brittleness. Brittleness can be reduced somewhat by tempering, with the sacrifice of some hardness. This is done by heating the previously quenched steel to some predetermined temperature. The illiterate sixteenth century master bladesmith tempered a quenched and hardened blade by the following steps:
a. Remove the oxide scale from the blade to expose bare metal.
b. Gently heat the blade until a colored oxide layer forms on the bare metal.
c. Remove the blade from the heat when the desired color is seen.
This works because the color of the oxide layer is a good indicator of the temperature of the steel and more importantly, because, that bladesmith served a lengthy apprenticeship under another illiterate master. Another example of knowing how without knowing why. Table I shows temperature versus oxide color. No, it's not an exact science. Go to a modem heat treatment facility and they get the same results but with better control due to greater knowledge. They simply stick the work piece in an oven thermostatically set to the desired temperature. Tables 2, 3, & 4 show hardness versus tempering temperature for different types of steel.
How long do you temper the blade? The answer is the same one that my wife gives when someone asks her “How long do you bake your bread?” She sighs deeply and says “Until it’s done.” All you have to do is bring the blade up to the desired temperature. No longer. Remember, you can ruin the temper on a knife blade in a few seconds by grinding on it until you get a blue oxide color.
It is often desirable to have the back of a knife blade softer than the cutting edge. The Japanese samurai did not want a sword that broke in ha1f in the middle of a battle, It was, however, acceptable to have a small section of the hardened edge break out without destroying the entire sword. Another reason for differential tempering is for rectifying a warped blade. If the blade comes out of the quenching bath with some warping it is impossible to straighten it in the full hard or moderately tempered state without breaking it. If you do sufficient differential tempering by softening the back of the blade then you can pound on the blade and get rid of the warp. My experience:
a. Polish the warped blade with emery cloth so the bare metal is exposed and you will be able to observe the oxide color.
b. Keep the cutting edge cool by contact with water or a wet rag.
c. Go over the back of the blade with a Bernzomatic torch until you see a blue oxide film. Do this in a place with good illumination so you can see the color change.
d. Beat on the blade with a wood mallet to remove the warp.
How Hard is Hard Enough?
We seek different hardness for different applications. Straight razors have to be sharp and hold an edge. They don't have to chop down trees. Consequently they are made from very hard brittle steel. I once knew a barber who collected old straight razors. He accidentally dropped one on the floor and it shattered. From that information I would deduce that a straight razor would be tempered at a very low temperature if at all. Wood carving is another activity that demands very sharp edges that don't get dull quickly. Wood carvers expect high hardness in there knives, typically Rc 62. People with tattooed biceps and camo sweatbands who teach wilderness survival want something that is more robust than the wood carvers and the knives they use are around Rc 58-60. Edge holding capability is very subjective and not easy to quantify.
Developing a Hamon Line
Much of the beauty seen in Japanese swords is contained in the hamon line, the transition created between the hardened edge and the very soft back of the blade. The traditional technique for this type of differential tempering is to use a very thick layer of clay on the part that is to remain soft. The results are a moderately hard cutting edge and a very soft back.
My first attempt at achieving a hamon line via differential tempering was done with 1095 steel and the following steps:
1. Heat blade to medium red heat.
2. Quench it in oil.
3. Temper entire blade at 375 F.
4. Remove oxide layer to expose bare metal.
5. Hold edge of blade against wet rag.
6. Heat back of blade with a pencil flame from a Bernzomatic torch until a dark blue oxide layer forms (550 deg F).
7. Polish the blade to expose bare metal.
The differential tempering was successful in that it was impossible to scratch the area near the edge with a file but the part with the blue oxide layer could be scratched. From Table 1 we assume that the dark blue oxide layer indicates a temperature of 550 deg F and from Table 4 the hardness is Rc 57. Next, I polished the blade but saw no visible hamon line transition between hard and moderately soft steel. I guessed that the part tempered to dark blue was not soft enough to create a hamon line.
In the second experiment, differential hardening, was done on a piece of scrap 1095 steel, I heated it red hot and then only part way immersed it in water. As a consequence there was an abrupt transition between very hard and very soft. Polishing revealed a hamon line. From these two experiments I conclude that there must be a transition from very hard to very soft in order to obtain a hamon line. This is verified by some metallurgical work done on a blade made by Don Fogg. In a report, http://www.dfoggknives.com/Metallurgical/METALLURGICAL%20REPORT.htm, the hardness near the cutting edge was measured to be Rc 61.2 and the hardness near the back of the blade was Rc 29.6. This is substantiated by a warning given to me by an avocational sword polisher to always slide a Japanese blade into its scabbard by contacting the back of the blade. Otherwise you run the risk of scratching the blade. From all this I conclude that in order to obtain a visible hamon line there must be a great difference in hardness between the two regions. Is it a tool, a weapon or an esthetic work of art? If the back of the blade is so soft that there is risk of scratching then it is not a practical tool and is more of an art object. There is no doubt or debate regarding the beauty and esthetic value of a blade with a visible hamon line but what about its functionality? Would it be more robust if the back had been tempered to a hardness of Rc55?
Conclusions
The next time you shave, chop wood or use a hacksaw take time to give thanks to the those early illiterate metal smiths who tried and tried until they got it right and gave us the recipe for heat treated steel.
Table 1
Temperature versus oxide color, from http://www.tpub.com/steelworker1/11.htm
Color |
Temperature |
Pale yellow |
428 220 |
Straw |
446 230 |
Golden yellow |
469 243 |
Brown |
491 255 |
Brown dappled with purple |
509 265 |
Purple |
531 277 |
Dark blue |
550 288 |
Bright blue |
567 297 |
Pale blue |
610 321 |
Table 2
From the Precision Steel Warehouse 1990 catalog:
Type |
1050 |
1095 |
Hardening Temp |
1500-1550 deg F |
1440-1475 deg F |
Tempering Temp: |
Rc Tensile psi |
Rc Tensile psi |
400 deg |
52 250,000 |
62 320,000 |
600 deg |
45 210,000 |
55 270,000 |
700 deg |
39 180,000 |
49 238,000 |
Table 3
From http://www.threeplanes.net/toolsteel.html
Hardness vs Tempering Temperature, O-1 tool steel
Tempering Temperature °F |
Tempering Temperature |
Approximate Hardness |
300 |
149 |
63/65 |
350 |
177 |
62/64 |
400 |
204 |
61/63 |
450 |
232 |
60/62 |
500 |
260 |
58/60 |
600 |
316 |
55/57 |
700 |
371 |
51/53 |
800 |
427 |
48/40 |
900 |
482 |
44/47 |
1000 |
538 |
40/44 |
Table 4
From http://tidewaterblacksmiths.net/2.html
Hardness vs. Tempering Temperature for Various Steels
Steel |
W 1 |
1080 |
01 |
4140 |
5160 |
A2 |
S7 |
H13 |
|
1095 |
|
|
4340 |
|
|
|
|
Tempering Temperature |
Hardness Rc |
Hardness Rc |
Hardness Rc |
Hardness Rc |
Hardness Rc |
Hardness Rc |
Hardness Rc |
Hardness Rc |
As quenched |
67 |
60 |
66 |
56 |
62 |
64 |
60 |
53 |
300 F |
64 |
59 |
63 |
53 |
60 |
62 |
59 |
52 |
400 F |
61 |
57 |
60 |
52 |
59 |
60 |
58 |
|
500F |
59 |
55 |
57 |
48 |
57 |
56 |
56 |
|
600F |
55 |
51 |
54 |
46 |
54 |
56 |
55 |
|
700F |
|
|
|
43 |
|
56 |
54 |
|
800F |
|
|
|
40 |
49 |
56 |
53 |
|
900F |
|
|
|
|
|
56 |
52 |
53 |
1000F |
|
|
|
|
38 |
56 |
51 |
54 |
1100F |
|
|
|
|
|
50 |
|
54 |
Figure 1
An attempt at water quenching a thin piece of 1095 steel.

Figure 2
A contemporary blade made by Walter Sorrells showing a beautiful hamon line. More details found at http//www.waltersorrells.com/blades/gallery.htm
E-mail your comments to "Richard A. Baugh" at richardbaugh@att.net
We hope the information on the PrimitiveWays website is both instructional and enjoyable. Understand that no warranty or guarantee is included. We expect adults to act responsibly and children to be supervised by a responsible adult. If you use the information on this site to create your own projects or if you try techniques described on PrimitiveWays, behave in accordance with applicable laws, and think about the sustainability of natural resources. Using tools or techniques described on PrimitiveWays can be dangerous with exposure to heavy, sharp or pointed objects, fire, stone tools and hazards present in outdoor settings. Without proper care and caution, or if done incorrectly, there is a risk of property damage, personal injury or even death. So, be advised: Anyone using any information provided on the PrimitiveWays website assumes responsibility for using proper care and caution to protect property, the life, health and safety of himself or herself and all others. He or she expressly assumes all risk of harm or damage to all persons or property proximately caused by the use of this information.
© PrimitiveWays 2013